by Nicholas L Balderston
Anxiety disorders are among the most diagnosed classes of mental disorders with 1 in 5 individuals meeting criteria for an anxiety disorder within a given year in the US (Kessler & Chiu, 2005). Critically, nearly 2/3 of anxiety patients rate their anxiety as moderate to severe, and less than half of anxiety patients receive minimally adequate treatment (Ginsburg et al., 2011). While anxiety alone can cause significant distress and dysfunction, anxiety and depression symptoms are highly comorbid, and those suffering from anxious depression are often much sicker and more difficult to treat than individuals with either depression or anxiety alone (Ionescu et al., 2014). For these reasons and others, anxiety disorders cost the US economy billions of dollars per year (Vos et al., 2020). Accordingly, it is critical that we develop novel treatments for anxiety.
Neuromodulation is rapidly emerging as a novel avenue for the treatment of psychiatric conditions, and transcranial magnetic stimulation (TMS), a noninvasive neuromodulation technique, has been shown to be a versatile tool in the arsenal (Lefaucheur et al., 2014). TMS works by creating a magnetic field that penetrates the skull and depolarizes neurons directly below the TMS coil (Box 1), leading to action potentials and plasticity in local circuits (Suppa et al., 2016). Critically, distinct patterns of TMS can be administered to either up- or down-regulate neural activity at the site of stimulation (Di Lazzaro et al., 2011), allowing researchers to target both hyper and hypo active circuits associated with a particular symptom or disorder. Additionally, TMS can be paired with cognitive tasks or behavioral cues meant to prime the circuits through Hebbian-like plasticity (Deng et al., 2020).
This flexibility has allowed researchers to develop several FDA-approved TMS treatments for depression (O’Reardon et al., 2007a), obsessive compulsive disorder (Carmi et al., 2019), and smoking cessation (Zangen et al., 2021), with several other applications currently in development. For depression there are 3 basic TMS treatments approved by the FDA. The first is the standard 10 Hz protocol targeting the left dorsolateral prefrontal cortex (dlPFC) (O’Reardon et al., 2007a). In this protocol, TMS is administered for ~40 min per day, 5 days per week, for roughly 4-6 weeks. This treatment has been shown to significantly reduce depression symptoms compared to sham (O’Reardon et al., 2007a). More recently, intermittent theta burst stimulation (iTBS) has been shown to be non-inferior to 10 Hz when given at the same intervals (Blumberger et al., 2018). The benefit to iTBS is that the stimulation sessions can be shortened from ~40 minutes to ~3.5 minutes without a drop in efficacy (Suppa et al., 2016). Finally, the most recently approved protocol the Stanford Neuromodulation Therapy (SNT) uses repeated daily sessions of iTBS (10 per day) to shorten the course of treatment from 4-6 weeks to 5 days with favorable outcomes (Cole et al., 2020, 2022).
Critically, all these treatment protocols target the left dlPFC. Mechanistically, it is thought that hypoconnectivity between the left dlPFC and the subgenual anterior cingulate cortex (sgACC) is thought to mediate the dysthymia experienced by those with depression (Sheline et al., 2009). Given the success of these treatments and the high comorbidity between depression and anxiety, researchers have begun to extend the use cases of these protocols to individuals with post-traumatic stress disorder (PTSD) (Kozel et al., 2018) and anxiety (Diefenbach, Bragdon, et al., 2016). While this approach has resulted in some limited success, it could be argued that decreases in anxiety following such treatments are secondary to the improvements in mood. According to this argument, treatments that target anxiety directly might be more effective at relieving anxiety symptoms. While there have been protocols developed to treat anxiety (Huang et al., 2018) and PTSD (Philip et al., 2019) symptoms, there is currently not sufficient clinical trial data to support widespread adoption of these protocols. Therefore, it is the purpose of this perspective to define a roadmap toward the FDA-approval and widespread adoption of a novel TMS treatment for anxiety/PTSD. In doing so, I will summarize the current support for existing protocols, as well as the additional support that would be needed to move these treatments forward.
For a medication or device to obtain approval from the FDA for a given indication, there needs to be sufficient scientific evidence that it is safe and effective for that indication. This evidence is typically the product of a yearslong series of increasingly large and well-controlled clinical trials that build upon the existing preclinical work identifying the mechanism of action. These trials are typically grouped into 1 of 4 phases based on their size and complexity.
Clinical Trials for PTSD and Anxiety
Discovery and Pre-Clinical Work
In general, preclinical work aims to discover novel therapeutics, and elucidate their mechanisms of action (Umscheid et al., 2011). Depending on the route, this may involve developments in chemistry, physics, genomics, computational biology, and biomedical engineering. These studies are often conducted in vitro, in silico, or in model organisms to minimize the risk to human subjects. For TMS, these data include studies of the effects of the strong magnetic pulses on the neurophysiology of neurons in the dish or the slice. These data also include work in humans targeting the motor system to understand how various experimental parameters affect cortical excitability.
Preclinically, much is known about the networks involved in anxiety and PTSD. Much of this work has been derived from studies of standard laboratory fear conditioning and extinction. In fear acquisition, an initially neutral stimulus like a picture or tone is repeatedly paired with an aversive stimulus like an electric shock (Kim & Jung, 2006; Lonsdorf et al., 2017). After repeated pairings, the previously neutral stimulus comes to elicit a fear response (Kim & Jung, 2006; Lonsdorf et al., 2017). In fear extinction, the pairing of the neutral and aversive stimulus is discontinued and the fear response to the previously neutral stimulus gradually diminishes (Fullana et al., 2018; Milad & Quirk, 2012). In addition to learning cue-outcome associations, the subject also learns that specific contexts or environments can become associated with a shock, which can lead to sustained anxiety responses in that context/environment (Maren et al., 2013).
Indeed, one of the benefits to studying anxiety is that the elevated physiological arousal that cuts across anxiety disorders is dimensional, experienced at varying levels in healthy individuals and patients alike, and readily studied using thoroughly researched and validated model systems (Grillon et al., 1994; Grillon, 2008a; Morgan et al., 1995; Robinson et al., 2012). Laboratory threat paradigms can also trigger mild anxiety symptoms not associated with arousal, like elevated subjective anxiety (Dunning et al., 2013; Hansen et al., 2009; Robinson, Vytal, et al., 2013; Vytal et al., 2014), worry (Grillon, 2002, 2008b), and attention control deficits. (Balderston et al., 2015; Clarke & Johnstone, 2013; Lavric et al., 2003; Robinson et al., 2011; Robinson, Krimsky, et al., 2013). It is therefore possible to use threat, with the substantial existing knowledge base of its underlying circuitry, to bridge the gap between pre-clinical work in non-human animals and anxiety patients and speed the discovery of new treatments.
Combined work in humans and non-human animals has led to the identification of a network of regions important for fear and anxiety. Critically, the amygdala is thought to be the key region where associations between events (neutral and aversive) are formed (Fanselow & Gale, 2003), leading to acute activation of downstream regions associated with behavioral outputs (hypothalamus, locus coeruleus, reticular formation, central grey, etc.) (Helmstetter et al., 2008; Ross & Van Bockstaele, 2021). In contrast, the bed nucleus of the stria terminalis (BNST) is thought to coordinate with the amygdala during sustained threats to prolong these behavioral responses (Davis et al., 2010). For more complex learning tasks, the hippocampus becomes important for linking temporally disconnected events (i.e. trace conditioning) or discriminating/generalizing details of an overall pattern that becomes associated with an aversive pattern (Gilmartin et al., 2014). The insula and primary sensory cortices all contribute to the experience of learned fear, while the dorsal anterior cingulate and motor cortices help coordinate defensive behaviors to learned threats (Fullana et al., 2015, 2018). Finally, the ventromedial anterior cingulate cortex (vmPFC; also known as sgACC) and the lateral prefrontal cortices are thought to be critical for learning extinction and for regulating fear and anxiety (Fullana et al., 2018; Milad & Quirk, 2012).
It is known that the hippocampus plays a critical role in episodic memory, spatial navigation, and pattern separation. For PTSD, there seems to be a direct link between symptom expression hippocampal function. It is known that chronic stress (Gurvits et al., 1996), sexual trauma (Bremner et al., 1997), and combat exposure (“MRI-Based Measurement of Hippocampal Volume in Patients with Combat- Related Posttraumatic Stress Disorder,” 1995) can reduce hippocampus volume and hippocampus neurogenesis in individuals with PTSD (Anacker & Hen, 2017). Individuals exposed with chronic stress are less able to recruit the hippocampus during memory retrieval (Carrion et al., 2010). Patients with PTSD show abnormal hippocampal functional connectivity, with disruptions in connections between the posterior hippocampus and default mode network (DMN) (Chen & Etkin, 2013). Together these results suggest that the hippocampus may be a critical target for intervention for PTSD.
Although there have been criticisms regarding the degree to which fear and anxiety translate to clinical anxiety symptoms, there is evidence that these paradigms relate to clinical phenotypes. First, patients with anxiety disorders have been shown to have elevated startle responses relative to control subjects (Grillon et al., 1994; Morgan et al., 1995), and this native startle potentiation increases as anxiety symptoms increase (Grillon, 2008b). Second, mirroring the increased prevalence of anxiety disorders in women, startle responses during unpredictable threat are also larger in women (Grillon, 2008a). Finally, these same startle responses are sensitive to the same anxiolytic drugs (e.g. benzodiazepines) as anxiety symptoms (Baas et al., 2002; Grillon et al., 2006; Rodríguez-Fornells et al., 1999; Scaife et al., 2005).
In addition to delineating potential targets and networks for TMS intervention, preclinical work with TMS has shed light on the mechanism of action of TMS generally (See Box 1), and rTMS/TBS specifically. Work examining the effect of repetitive TMS (rTMS) on cortical excitability suggests that patterns below 5 Hz tend to decrease excitability at the site of stimulation, while patterns greater than 5 Hz tend to increase excitability at the site of stimulation (Di Lazzaro et al., 2011). However, these effects are likely dependent upon several factors including the strength of stimulation, number of pulses, and others (Di Lazzaro et al., 2011). Theta burst stimulation mimics natural rhythms of the brain to efficiently modulate synaptic plasticity (Suppa et al., 2016), inducing behavioral changes in targeted circuits in a fraction of time as traditional rTMS. Distinct patterns of TBS can lead to distinct effects on plasticity. For most common protocols, iTBS tends to strengthen synaptic connections, while continuous TBS (cTBS) tends to weaken synaptic connections. Additionally, there is evidence that administering repeated sessions daily can further facilitate behavioral effects of TBS (Goldsworthy et al., 2015; Thomson & Sack, 2020).
Phase I Trials
These tend to be small (20-80 participants and are aimed at first-in-human tests of safety for a drug or device (Umscheid et al., 2011). They are also intended to identify any potential side effects of a drug or device. For TMS the safety and side effect profile has been well-established (Rossi et al., 2020). The primary side effects tend to involve discomfort at the site of stimulation and an elevated risk of seizure (Rossi et al., 2020). With new applications of TMS, there are typically several smaller trials typically conducted aimed at understanding how TMS might target a specific mechanism related to a disease or symptom. These trials typically also investigate approaches to optimize and individualize the targeting of such mechanisms using techniques like fMRI (Balderston, Roberts, et al., 2020) or connectivity (Balderston et al., 2022; Oathes et al., 2021) based TMS targeting.
The initial work examining the effect of TMS on anxiety stemmed from studies in individuals with a primary diagnosis of depression. In these individuals, evidence began to mount that 1 Hz stimulation to the right dlPFC could be anxiolytic (Mantovani et al., 2013; O’Reardon et al., 2007b; D. White & Tavakoli, 2015). Soon, groups began piloting 1 Hz stimulation protocols in generalized anxiety disorder (GAD) patients with some success. However, there have yet to be large-scale well-controlled trials targeting anxiety with this approach. Perhaps one reason for this lack of RCTs is the fact that the mechanism underlying this anxiolytic effect is largely unknown, making it difficult to identify the optimal right prefrontal target.
Other mechanistic work has used TMS as a causal intervention to probe frontal and parietal circuits and determine their involvement in anxiety expression and regulation. There is evidence from structural connectivity that the amygdala can be targeted via its functional connections with the ventrolateral prefrontal cortex (vlPFC) (Sydnor et al., 2022). TMS/EEG has shown that spider phobic individuals have reduced inhibition in the dlPFC when exposed to spider images, suggesting that fear and fear-relevant stimuli capture attention and attentional resources (Pokorny et al., 2022). There is evidence to suggest that 20 Hz stimulation to the left dlPFC, an area important for regulation, during extinction can enhance extinction retention (Raij et al., 2018) during standard laboratory delay fear conditioning. In contrast, low frequency stimulation to the dorsal anterior cingulate cortex (dACC), an area important for expression, reduced spontaneous recovery during trace fear conditioning (Klavir et al., 2012).
Work from my own lab uses threat of unpredictable shock to experimentally elevate state anxiety and arousal, along with concurrent anxiety ratings and anxiety potentiated startle (APS) to measure state anxiety and arousal in the laboratory (Grillon, 2008b). Using this approach, we have shown that both excitatory (10 Hz (Balderston, Beydler, Roberts, et al., 2020) and iTBS [unpublished]) and inhibitory (Teferi et al., 2022) (cTBS) protocols lead to increased anxiety (rather than decreased anxiety) when applied to the right dlPFC in healthy volunteers. This is true whether we measure changes in anxiety/arousal acutely (Balderston, Beydler, Roberts, et al., 2020) or after a 24-hour delay (Teferi et al., 2022), suggesting the effects are related to changes in plasticity. These effects have been replicated in studies targeting heart rate variability with dlPFC and frontotemporal iTBS (Notzon et al., 2015; Poppa et al., 2020). While it may be tempting to use these findings to argue against right dlPFC stimulation for anxiety, it is unclear whether our findings generalize to patients (L. K. White et al., 2023). Despite this limitation, our results confirm a mechanistic link between right dlPFC function and elevated arousal and provide an objective measure (APS) to study this link in patients.
A small study using deep TMS paired with exposure to a brief traumatic event showed that this paradigm can potentially lead to decreased PTSD symptoms (Isserles et al., 2013). 1 Hz rTMS to the right dlPFC reduced hyperarousal symptoms in a small crossover pilot with 9 subjects (Osuch et al., 2009). In a series of case studies, researchers were able to show that bilateral iTBS given once daily for 4 weeks (5 days per week) can reduce both depression and PTSD symptoms (Nursey et al., 2020). Retrospective analysis of medical records of PTSD patients treated with TMS suggests that TMS is effective in individuals with and without traumatic brain injury (Philip et al., 2023). Finally, TMS/EEG can predict clinical outcomes for PTSD patients who receive TMS, suggesting that there are electrophysiological signals associated with TMS treatment outcomes (Zandvakili et al., 2019).
Phase II Trials
These are typically conducted in a somewhat larger group of individuals (100-300 participants) and are aimed at further examining the safety and effectiveness of a given drug or device (Umscheid et al., 2011). With novel applications of TMS, these Phase II trials are also designed to optimize the parameters of the TMS protocol to deliver a treatment that safe, efficacious, and efficient. These trials tend to investigate the effect of TMS pattern (i.e. rTMS vs. theta burst), intended effect (excitatory vs. inhibitory), dose, timing, number of pulses, and number of sessions. These trials also give researchers an opportunity to identify and validate potential outcome measures for larger Phase III trials. For PTSD and anxiety, there have been several small to medium sized trials conducted examining the effect of lf-rTMS, hf-rTMS, and iTBS on PTSD and anxiety symptoms.
GAD. For GAD, there was a small trial aimed at right dlPFC with 13 active and 12 sham patients (Diefenbach, Bragdon, et al., 2016) showing that 30 sessions of active 1 Hz stimulation improves response and remission rates compared to sham. Active subjects also showed improved emotion regulation (Diefenbach, Assaf, et al., 2016). Another small trial with 28 subjects showed that bilateral 1 Hz stimulation can reduce anxiety ratings, but this trial lacked a sham control condition (Lu et al., 2018). Finally, one study targeting the right parietal cortex with 1 Hz stimulation showed decreased anxiety and insomnia symptoms in active compared to sham subjects (Huang et al., 2018). This is similar to our findings in healthy subjects that 1 Hz stimulation to the parietal cortex can reduce anxiety potentiated startle during threat of unpredictable shock (Balderston, Beydler, Goodwin, et al., 2020).
In terms of hf-rTMS, a small trial where 15 active subjects received 25 sessions of 20 Hz stimulation to the right dlPFC. This trial showed reduced Hamilton anxiety rating scores in the active group compared to the sham group, and these scores were stable at 1 month (Dilkov et al., 2017). A pair of retrospective trials looked at the effect of hf-rTMS administered via either a figure 8 coil to the left dlPFC (Caulfield & Stern, 2020) or via the H1 deep TMS coil (Pell et al., 2022) reduced anxiety ratings in GAD and GAD/MDD patients. Finally, there was an additional trial in GAD/MDD patients using 10 sessions of hf-rTMS to the left dlPFC showing a reduction in both depression and anxiety ratings. However, this trial lacked a sham comparison (Diefenbach et al., 2013).
PTSD. For PTSD, the largest lf-rTMS clinical trial I was able to find had 54 active and 49 sham subjects receiving 12-15 sessions of 1 Hz stimulation to the right dlPFC. They showed greater CAPS and PCL reductions in the active group compared to the sham, and these reductions were stable at a 6-month follow-up (Kozel et al., 2018). These results are consistent with 3 other small trials with between 10 and 20 subjects per group receiving similar courses of stimulation to the right dlPFC (Kozel et al., 2019; Leong et al., 2020; Nam et al., 2013). 5 Hz stimulation to the left dlPFC seems to also reduce MDD and PTSD symptoms, however it is unclear whether this treatment outperforms sham (Carpenter et al., 2018).
For hf-rTMS and PTSD, the results are mixed. There’s some evidence to suggest that either 10 Hz to the left (Wilkes et al., 2020) and 20 Hz to the left or right dlPFC (Boggio et al., 2010) could reduce PTSD symptoms. However, the trials showing the effect with 10 Hz stimulation was retrospective and lacked a sham control (Wilkes et al., 2020). In contrast, a recent moderately powered (125 total participants) trial examining the effect of 18 Hz dTMS found that the sham stimulation outperformed the active stimulation (Isserles et al., 2021). Like hf-rTMS, iTBS is thought to be excitatory. There is evidence from 2 recent iTBS trials suggesting that 10 session of iTBS reduces anger (van ’t Wout-Frank et al., 2021) and marginally improves PTSD symptoms compared to sham (Philip et al., 2019), and 20 sessions can lead to stable reductions in PTSD symptoms lasting up to 1 year (Petrosino et al., 2020). Additionally, machine learning work with TMS-evoked EEG responses suggests that these responses carry important information about who will benefit from treatment and who will not (Zandvakili et al., 2021).
Phase III Trials
Once a basic clinical effect has been established in a Phase II trial, the new drug/device will be tested in a large group of people (1,000–3,000 participants) in a Phase III trials (Umscheid et al., 2011). These trials are typically designed to confirm the results of the Phase II trial, check for side effects, or compare the experimental drug/device to existing treatments. In TMS, these typically also involve a comparison between active and sham stimulation, randomization to treatment arms, and blinding of both the participants and the study staff. Pending completion of a successful Phase III randomized controlled clinical trial, there is generally sufficient evidence to apply for FDA approval for the TMS protocol under study. Unfortunately, there has yet to be any sufficiently large, well-controlled study using TMS for anxiety or PTSD.
Future Directions
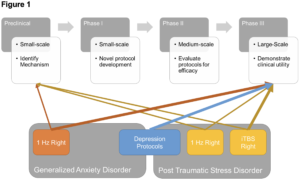
As shown above, there are 3 primary limitations impeding our path to an FDA approved neuromodulatory treatment for anxiety. First, while we have learned much about the how anxiety and fear are expressed and regulated in the brain through the preclinical and Phase 1 trials above, we still lack a basic understanding of how to translate this knowledge into an optimal protocol. Second, while there have been several small-scale trials conducted in PTSD and anxiety patients, the target and pattern of stimulation varies across these trials, further supporting our need for a clear mechanistic target. Third, there has yet to be any large-scale, randomized, blinded, sham-controlled clinical trials using TMS to treat PTSD or anxiety.
Without a clear mechanistic target to guide the neuromodulatory research into PTSD and anxiety treatments, the strategy in the field has been to adapt protocols used in depression to these additional populations. This makes sense given the high comorbidity between depression symptoms and fear/anxiety symptoms. It follows then that this approach has led to multiple, small somewhat successful trials (described above). Perhaps there is a unitary dimension of negative mood that encompasses the shared variability between depression and fear/anxiety that is successfully treated by targeting the dlPFC (Mantovani et al., 2013; O’Reardon et al., 2007b; D. White & Tavakoli, 2015). To take it a step further, perhaps standard FDA approved depression protocols should be the first line of TMS treatments for all mood disorders, with a second line of add-on or follow-up protocols aimed at other orthogonal symptom dimensions.
Going forward, I argue that the clearest most efficacious pathway may be to 1) conduct large scale RCTs using depression protocols in PTSD and anxiety. 2) Identify a single or set of symptom dimensions that capture the shared variability between PTSD and anxiety (e.g., elevated arousal, startle reactivity, hypervigilance etc.). 3) Conduct several smaller, well-controlled transdiagnostic studies targeted at reducing expression along this symptom dimension. 4) Select from these transdiagnostic studies the most promising novel treatment protocols and test them with large-scale Phase III trials (See Figure 1). Such an approach would capitalize on the wealth of knowledge acquired from studying MDD and large comorbidity shared across mood disorders, while also yielding new information specific to fear and anxiety that could generate novel second-line treatments or patients who do not benefit from the traditional protocols.
Acknowledgments: The authors would like to thank Maria Prociuk for her expertise and assistance in submitting the paper.
Disclosures: This project was supported in part by a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation (NLB: 2021); and by a U01 award U01MH130447 (NLB). The authors report no biomedical financial interests or potential conflicts of interest.
References
Anacker, C., & Hen, R. (2017). Adult hippocampal neurogenesis and cognitive flexibility—Linking memory and mood. Nature Reviews Neuroscience, 18(6), 335–346. https://doi.org/10.1038/nrn.2017.45
Baas, J. M., Grillon, C., Böcker, K. B., Brack, A. a., Morgan, C. a., Kenemans, L. J., & Verbaten, M. N. (2002). Benzodiazepines have no effect on fear-potentiated startle in humans. Psychopharmacology, 161(3), 233–247. https://doi.org/10.1007/s00213-002-1011-8
Balderston, N. L., Beer, J. C., Seok, D., Makhoul, W., Deng, Z.-D. D., Girelli, T., Teferi, M., Smyk, N., Jaskir, M., Oathes, D. J., & Sheline, Y. I. (2022). Proof of concept study to develop a novel connectivity-based electric-field modelling approach for individualized targeting of transcranial magnetic stimulation treatment. Neuropsychopharmacology, 47(2), Article 2. https://doi.org/10.1038/s41386-021-01110-6
Balderston, N. L., Beydler, E. M. E. M., Roberts, C., Deng, Z.-D. D., Radman, T., Lago, T., Luber, B. M., Lisanby, S. H., Ernst, M., & Grillon, C. (2020). Mechanistic link between right prefrontal cortical activity and anxious arousal revealed using transcranial magnetic stimulation in healthy subjects. Neuropsychopharmacology, 45(4), Article 4. https://doi.org/10.1038/s41386-019-0583-5
Balderston, N. L., Beydler, E. M., Goodwin, M., Deng, Z.-D., Radman, T., Luber, B. M., Lisanby, S. H., Ernst, M., & Grillon, C. (2020). Low-frequency parietal repetitive transcranial magnetic stimulation reduces fear and anxiety. Translational Psychiatry, 10(1), Article 1. https://doi.org/10.1038/s41398-020-0751-8
Balderston, N. L., Mathur, A., Adu-Brimpong, J., Hale, E. A., Ernst, M., & Grillon, C. (2015). Effect of anxiety on behavioural pattern separation in humans. Cognition and Emotion, 9931(October), 1–11. https://doi.org/10.1080/02699931.2015.1096235
Balderston, N. L., Roberts, C., Beydler, E. M., Deng, Z.-D., Radman, T., Luber, B. M., Lisanby, S. H., Ernst, M., & Grillon, C. (2020). A generalized workflow for conducting electric field–optimized, fMRI-guided, transcranial magnetic stimulation. Nature Protocols, 15(11), Article 11. https://doi.org/10.1038/s41596-020-0387-4
Blumberger, D. M., Vila-Rodriguez, F., Thorpe, K. E., Feffer, K., Noda, Y., Giacobbe, P., Knyahnytska, Y., Kennedy, S. H., Lam, R. W., Daskalakis, Z. J., & Downar, J. (2018). Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): A randomised non-inferiority trial. The Lancet, 391(10131), Article 10131. https://doi.org/10.1016/S0140-6736(18)30295-2
Boggio, P. S., Rocha, M., Oliveira, M. O., Fecteau, S., Cohen, R. B., Campanhã, C., Ferreira-Santos, E., Meleiro, A., Corchs, F., Zaghi, S., Pascual-Leone, A., & Fregni, F. (2010). Noninvasive brain stimulation with high-frequency and low-intensity repetitive transcranial magnetic stimulation treatment for posttraumatic stress disorder. Journal of Clinical Psychiatry, 71(8), Article 8. https://doi.org/10.4088/JCP.08m04638blu
Bremner, J. D., Randall, P., Vermetten, E., Staib, L., Bronen, R. A., Mazure, C., Capelli, S., McCarthy, G., Innis, R. B., & Charney, D. S. (1997). Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse—A preliminary report. Biological Psychiatry, 41(1), 23–32. https://doi.org/10.1016/S0006-3223(96)00162-X
Carmi, L., Tendler, A., Bystritsky, A., Hollander, E., Blumberger, D. M., Daskalakis, J., Ward, H., Lapidus, K., Goodman, W., Casuto, L., Feifel, D., Barnea-Ygael, N., Roth, Y., Zangen, A., & Zohar, J. (2019). Efficacy and Safety of Deep Transcranial Magnetic Stimulation for Obsessive-Compulsive Disorder: A Prospective Multicenter Randomized Double-Blind Placebo-Controlled Trial. The American Journal of Psychiatry, 176(11), 931–938. https://doi.org/10.1176/appi.ajp.2019.18101180
Carpenter, L. L., Conelea, C., Tyrka, A. R., Welch, E. S., Greenberg, B. D., Price, L. H., Niedzwiecki, M., Yip, A. G., Barnes, J., & Philip, N. S. (2018). 5 Hz Repetitive transcranial magnetic stimulation for posttraumatic stress disorder comorbid with major depressive disorder. Journal of Affective Disorders, 235(February), Article February. https://doi.org/10.1016/j.jad.2018.04.009
Carrion, V. G., Haas, B. W., Garrett, A., Song, S., & Reiss, A. L. (2010). Reduced Hippocampal Activity in Youth with Posttraumatic Stress Symptoms: An fMRI Study. Journal of Pediatric Psychology, 35(5), 559–569. https://doi.org/10.1093/jpepsy/jsp112
Caulfield, K. A., & Stern, A. P. (2020). Therapeutic High-Frequency Repetitive Transcranial Magnetic Stimulation Concurrently Improves Mood and Anxiety in Patients Using Benzodiazepines. Neuromodulation, 23(3), Article 3. https://doi.org/10.1111/ner.13024
Chen, A. C., & Etkin, A. (2013). Hippocampal network connectivity and activation differentiates post-traumatic stress disorder from generalized anxiety disorder. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 38(10), Article 10. https://doi.org/10.1038/npp.2013.122
Clarke, R., & Johnstone, T. (2013). Prefrontal inhibition of threat processing reduces working memory interference. Frontiers in Human Neuroscience, 7(May), 228. https://doi.org/10.3389/fnhum.2013.00228
Cole, E. J., Phillips, A. L., Bentzley, B. S., Stimpson, K. H., Nejad, R., Barmak, F., Veerapal, C., Khan, N., Cherian, K., Felber, E., Brown, R., Choi, E., King, S., Pankow, H., Bishop, J. H., Azeez, A., Coetzee, J., Rapier, R., Odenwald, N., … Williams, N. R. (2022). Stanford Neuromodulation Therapy (SNT): A Double-Blind Randomized Controlled Trial. The American Journal of Psychiatry, 179(2), Article 2. https://doi.org/10.1176/appi.ajp.2021.20101429
Cole, E. J., Stimpson, K. H., Bentzley, B. S., Gulser, M., Cherian, K., Tischler, C., Nejad, R., Pankow, H., Choi, E., Aaron, H., Espil, F. M., Pannu, J., Xiao, X., Duvio, D., Solvason, H. B., Hawkins, J., Guerra, A., Jo, B., Raj, K. S., … Williams, N. R. (2020). Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. American Journal of Psychiatry, 177(8), Article 8. https://doi.org/10.1176/appi.ajp.2019.19070720
Davis, M., Walker, D. L., Miles, L., & Grillon, C. (2010). Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 35(1), Article 1. https://doi.org/10.1038/npp.2009.109
Deng, Z.-D., Luber, B. M., Balderston, N. L., Velez Afanador, M., Noh, M. M. M., Thomas, J., Altekruse, W. C. W. C., Exley, S. L. S. L., Awasthi, S., & Lisanby, S. H. (2020). Device-Based Modulation of Neurocircuits as a Therapeutic for Psychiatric Disorders. Annual Review of Pharmacology and Toxicology, 60(1), Article 1. https://doi.org/10.1146/annurev-pharmtox-010919-023253
Di Lazzaro, V., Dileone, M., Pilato, F., Capone, F., Musumeci, G., Ranieri, F., Ricci, V., Bria, P., Di Iorio, R., de Waure, C., Pasqualetti, P., & Profice, P. (2011). Modulation of motor cortex neuronal networks by rTMS: comparison of local and remote effects of six different protocols of stimulation. Journal of Neurophysiology, 105(5), 2150–2156. https://doi.org/10.1152/jn.00781.2010
Diefenbach, G. J., Assaf, M., Goethe, J. W., Gueorguieva, R., & Tolin, D. F. (2016). Improvements in emotion regulation following repetitive transcranial magnetic stimulation for generalized anxiety disorder. Journal of Anxiety Disorders, 43, 1–7. https://doi.org/10.1016/j.janxdis.2016.07.002
Diefenbach, G. J., Bragdon, L. B., Zertuche, L., Hyatt, C. J., Hallion, L. S., Tolin, D. F., Goethe, J. W., & Assaf, M. (2016). Repetitive transcranial magnetic stimulation for generalised anxiety disorder: A pilot randomised, double-blind, sham-controlled trial. British Journal of Psychiatry, 209(3), Article 3. https://doi.org/10.1192/bjp.bp.115.168203
Diefenbach, G. J., Bragdon, L., & Goethe, J. W. (2013). Treating anxious depression using repetitive transcranial magnetic stimulation. Journal of Affective Disorders, 151(1), 365–368. https://doi.org/10.1016/j.jad.2013.05.094
Dilkov, D., Hawken, E. R., Kaludiev, E., & Milev, R. (2017). Repetitive transcranial magnetic stimulation of the right dorsal lateral prefrontal cortex in the treatment of generalized anxiety disorder: A randomized, double-blind sham controlled clinical trial. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 78(September 2016), Article September 2016. https://doi.org/10.1016/j.pnpbp.2017.05.018
Dunning, J. P., Deldonno, S., & Hajcak, G. (2013). The effects of contextual threat and anxiety on affective startle modulation. Biological Psychology, 94(1), 130–135. https://doi.org/10.1016/j.biopsycho.2013.05.013
Fanselow, M. S., & Gale, G. D. (2003). The amygdala, fear, and memory. Annals of the New York Academy Of, 985, 125–134.
Fullana, M. A., Albajes-Eizagirre, A., Soriano-Mas, C., Vervliet, B., Cardoner, N., Benet, O., Radua, J., & Harrison, B. J. (2018). Fear extinction in the human brain: A meta-analysis of fMRI studies in healthy participants. Neuroscience and Biobehavioral Reviews, 88, 16–25. https://doi.org/10.1016/j.neubiorev.2018.03.002
Fullana, M. A., Harrison, B. J., Soriano-Mas, C., Vervliet, B., Cardoner, N., Àvila-Parcet, A., & Radua, J. (2015). Neural signatures of human fear conditioning: An updated and extended meta-analysis of fMRI studies. Molecular Psychiatry, 21(April 2015), Article April 2015. https://doi.org/10.1038/mp.2015.88
Gilmartin, M. R., Balderston, N. L., & Helmstetter, F. J. (2014). Prefrontal cortical regulation of fear learning. Trends in Neurosciences, 37(8), Article 8. https://doi.org/10.1016/j.tins.2014.05.004
Ginsburg, G. S., Kendall, P. C., Sakolsky, D., Compton, S. N., Piacentini, J., Albano, A. M., Walkup, J. T., Sherrill, J., Coffey, K. A., Rynn, M. A., Keeton, C. P., McCracken, J. T., Bergman, L., Iyengar, S., Birmaher, B., & March, J. (2011). Remission after acute treatment in children and adolescents with anxiety disorders: Findings from the CAMS. Journal of Consulting and Clinical Psychology, 79(6), 806–813. https://doi.org/10.1037/a0025933
Goldsworthy, M. R., Müller-Dahlhaus, F., Ridding, M. C., & Ziemann, U. (2015). Resistant Against De-depression: LTD-Like Plasticity in the Human Motor Cortex Induced by Spaced cTBS. Cerebral Cortex, 25(7), 1724–1734. https://doi.org/10.1093/cercor/bht353
Grillon, C. (2002). Startle reactivity and anxiety disorders: Aversive conditioning, context, and neurobiology. Biological Psychiatry, 52(10), 958–975. https://doi.org/10.1016/S0006-3223(02)01665-7
Grillon, C. (2008a). Greater sustained anxiety but not phasic fear in women compared to men. Emotion (Washington, D.C.), 8(3), 410–413. https://doi.org/10.1037/1528-3542.8.3.410
Grillon, C. (2008b). Models and mechanisms of anxiety: Evidence from startle studies. Psychopharmacology, 199, 421–437. https://doi.org/10.1007/s00213-007-1019-1
Grillon, C., Ameli, R., Goddard, A., Woods, S. W., & Davis, M. (1994). Baseline and fear-potentiated startle in panic disorder patients. Biological Psychiatry, 35(7), 431–439. https://doi.org/10.1016/0006-3223(94)90040-X
Grillon, C., Baas, J. M. P., Pine, D. S., Lissek, S., Lawley, M., Ellis, V., & Levine, J. (2006). The Benzodiazepine Alprazolam Dissociates Contextual Fear from Cued Fear in Humans as Assessed by Fear-potentiated Startle. Biological Psychiatry, 60(7), 760–766. https://doi.org/10.1016/j.biopsych.2005.11.027
Gurvits, T. V., Shenton, M. E., Hokama, H., Ohta, H., Lasko, N. B., Gilbertson, M. W., Orr, S. P., Kikinis, R., Jolesz, F. A., McCarley, R. W., & Pitman, R. K. (1996). Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biological Psychiatry, 40(11), 1091–1099. https://doi.org/10.1016/S0006-3223(96)00229-6
Hansen, A. L., Johnsen, B. H., & Thayer, J. F. (2009). Relationship between heart rate variability and cognitive function during threat of shock. Anxiety, Stress, and Coping, 22(1), 77–89. https://doi.org/10.1080/10615800802272251
Helmstetter, F. J., Parsons, R. G., & Gafford, G. M. (2008). Macromolecular synthesis, distributed synaptic plasticity, and fear conditioning. Neurobiology of Learning and Memory, 89(3), Article 3. https://doi.org/10.1016/j.nlm.2007.09.002
Huang, Z., Li, Y., Bianchi, M. T., Zhan, S., Jiang, F., Li, N., Ding, Y., Hou, Y., Wang, L., Ouyang, Q., & Wang, Y. (2018). Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: A randomized, double-blind, sham-controlled pilot study. Brain Stimulation, 11(5), Article 5. https://doi.org/10.1016/j.brs.2018.05.016
Ionescu, D. F., Niciu, M. J., Richards, E. M., & Zarate, C. A. (2014). Pharmacologic treatment of dimensional anxious depression: A review. The Primary Care Companion for CNS Disorders, 16(3), PCC.13r01621. https://doi.org/10.4088/PCC.13r01621
Isserles, M., Shalev, A. Y., Roth, Y., Peri, T., Kutz, I., Zlotnick, E., & Zangen, A. (2013). Effectiveness of deep transcranial magnetic stimulation combined with a brief exposure procedure in post-traumatic stress disorder-a pilot study. Brain Stimulation, 6(3), Article 3. https://doi.org/10.1016/j.brs.2012.07.008
Isserles, M., Tendler, A., Roth, Y., Bystritsky, A., Blumberger, D. M., Ward, H., Feifel, D., Viner, L., Duffy, W., Zohar, J., Keller, C. J., Bhati, M. T., Etkin, A., George, M. S., Filipcic, I., Lapidus, K., Casuto, L., Vaishnavi, S., Stein, A., … Ressler, K. J. (2021). Deep Transcranial Magnetic Stimulation Combined With Brief Exposure for Posttraumatic Stress Disorder: A Prospective Multisite Randomized Trial. Biological Psychiatry, 90(10), 721–728. https://doi.org/10.1016/j.biopsych.2021.04.019
Kessler, R., & Chiu, W. (2005). Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General …, 62(6), 617–627. https://doi.org/10.1001/archpsyc.62.6.617.Prevalence
Kim, J. J., & Jung, M. W. (2006). Neural circuits and mechanisms involved in Pavlovian fear conditioning: A critical review. Neuroscience and Biobehavioral Reviews, 30(2), Article 2. https://doi.org/10.1016/j.neubiorev.2005.06.005
Klavir, O., Genud-Gabai, R., & Paz, R. (2012). Low-Frequency Stimulation Depresses the Primate Anterior-Cingulate-Cortex and Prevents Spontaneous Recovery of Aversive Memories. Journal of Neuroscience, 32(25), 8589–8597. https://doi.org/10.1523/JNEUROSCI.6481-11.2012
Kozel, F. A., Motes, M. A., Didehbani, N., DeLaRosa, B., Bass, C., Schraufnagel, C. D., Jones, P., Morgan, C. R., Spence, J. S., Kraut, M. A., & Hart, J. (2018). Repetitive TMS to augment cognitive processing therapy in combat veterans of recent conflicts with PTSD: A randomized clinical trial. Journal of Affective Disorders, 229(July 2017), Article July 2017. https://doi.org/10.1016/j.jad.2017.12.046
Kozel, F. A., Van Trees, K., Larson, V., Phillips, S., Hashimie, J., Gadbois, B., Johnson, S., Gallinati, J., Barrett, B., Toyinbo, P., Weisman, M., Centorino, M., Gibson, C. A., & Catalano, G. (2019). One hertz versus ten hertz repetitive TMS treatment of PTSD: A randomized clinical trial. Psychiatry Research, 273(December 2018), Article December 2018. https://doi.org/10.1016/j.psychres.2019.01.004
Lavric, A., Rippon, G., & Gray, J. R. (2003). Threat-evoked anxiety disrupts spatial working memory performance: An attentional account. Cognitive Therapy and Research, 27(5), 489–504. https://doi.org/10.1023/A:1026300619569
Lefaucheur, J. P., Andre-Obadia, N., Antal, A., Ayache, S. S., Baeken, C., Benninger, D. H., Cantello, R. M., Cincotta, M., de Carvalho, M., De Ridder, D., Devanne, H., Di Lazzaro, V., Filipovic, S. R., Hummel, F. C., Jaaskelainen, S. K., Kimiskidis, V. K., Koch, G., Langguth, B., Nyffeler, T., … Garcia-Larrea, L. (2014). Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol, 125(11), Article 11. https://doi.org/S1388-2457(14)00296-X [pii]\r10.1016/j.clinph.2014.05.021 [doi]
Leong, K., Chan, P., Ong, L., Zwicker, A., Willan, S., Lam, R. W., & McGirr, A. (2020). A Randomized Sham-controlled Trial of 1-Hz and 10-Hz Repetitive Transcranial Magnetic Stimulation (rTMS) of the Right Dorsolateral Prefrontal Cortex in Civilian Post-traumatic Stress Disorder: Un essai randomisé contrôlé simulé de stimulation magnétique t. Canadian Journal of Psychiatry, 65(11), Article 11. https://doi.org/10.1177/0706743720923064
Lonsdorf, T. B., Menz, M. M., Andreatta, M., Fullana, M. A., Golkar, A., Haaker, J., Heitland, I., Hermann, A., Kuhn, M., Kruse, O., Meir Drexler, S., Meulders, A., Nees, F., Pittig, A., Richter, J., Römer, S., Shiban, Y., Schmitz, A., Straube, B., … Merz, C. J. (2017). Don ’ t fear ‘ fear conditioning ’: Methodological considerations for the design and analysis of … Neuroscience & Biobehavioral Reviews, 77(March), Article March. https://doi.org/10.1016/j.neubiorev.2017.02.026
Lu, R., Zhang, C., Liu, Y., Wang, L., Chen, X., & Zhou, X. (2018). The effect of bilateral low-frequency rTMS over dorsolateral prefrontal cortex on serum brain-derived neurotropic factor and serotonin in patients with generalized anxiety disorder. Neuroscience Letters, 684, 67–71. https://doi.org/10.1016/j.neulet.2018.07.008
Mantovani, A., Aly, M., Dagan, Y., Allart, A., & Lisanby, S. H. (2013). Randomized sham controlled trial of repetitive transcranial magnetic stimulation to the dorsolateral prefrontal cortex for the treatment of panic disorder with comorbid major depression. Journal of Affective Disorders, 144(1–2), 153–159. https://doi.org/10.1016/j.jad.2012.05.038
Maren, S., Phan, K. L., & Liberzon, I. (2013). The contextual brain: Implications for fear conditioning, extinction and psychopathology. Nature Reviews Neuroscience, 14(6), Article 6. https://doi.org/10.1038/nrn3492
Milad, M. R., & Quirk, G. J. (2012). Fear extinction as a model for translational neuroscience: Ten years of progress. Annual Review of Psychology, 63, 129–151. https://doi.org/10.1146/annurev.psych.121208.131631
Morgan, C. a, Grillon, C., Southwick, S. M., Davis, M., & Charney, D. S. (1995). Fear-potentiated startle in posttraumatic stress disorder. Biological Psychiatry, 38(6), 378–385. https://doi.org/10.1016/0006-3223(94)00321-S
MRI-based measurement of hippocampal volume in patients with combat- related posttraumatic stress disorder. (1995). American Journal of Psychiatry, 152(7), 973–981. https://doi.org/10.1176/ajp.152.7.973
Nam, D. H., Pae, C. U., & Chae, J. H. (2013). Low-frequency, repetitive transcranial magnetic stimulation for the treatment of patients with posttraumatic stress disorder: A double-blind, sham-controlled study. Clinical Psychopharmacology and Neuroscience, 11(2), Article 2. https://doi.org/10.9758/cpn.2013.11.2.96
Notzon, S., Deppermann, S., Fallgatter, A., Diemer, J., Kroczek, A., Domschke, K., Zwanzger, P., & Ehlis, A.-C. (2015). Psychophysiological effects of an iTBS modulated virtual reality challenge including participants with spider phobia. Biological Psychology, 112, 66–76. https://doi.org/10.1016/j.biopsycho.2015.10.003
Nursey, J., Sbisa, A., Knight, H., Ralph, N., Cowlishaw, S., Forbes, D., O’Donnell, M., Hinton, M., Cooper, J., Hopwood, M., McFarlane, A., Herring, S., & Fitzgerald, P. (2020). Exploring Theta Burst Stimulation for Post-traumatic Stress Disorder in Australian Veterans—A Pilot Study. Military Medicine, 185(9–10), e1770–e1778. https://doi.org/10.1093/milmed/usaa149
Oathes, D. J., Zimmerman, J. P., Duprat, R., Japp, S. S., Scully, M., Rosenberg, B. M., Flounders, M. W., Long, H., Deluisi, J. A., Elliott, M., Shandler, G., Shinohara, R. T., & Linn, K. A. (2021). Resting fMRI-guided TMS results in subcortical and brain network modulation indexed by interleaved TMS/fMRI. Experimental Brain Research, 239(4), Article 4. https://doi.org/10.1007/s00221-021-06036-5
O’Reardon, J. P., Solvason, H. B., Janicak, P. G., Sampson, S., Isenberg, K. E., Nahas, Z., McDonald, W. M., Avery, D., Fitzgerald, P. B., Loo, C., Demitrack, M. A., George, M. S., & Sackeim, H. A. (2007a). Efficacy and Safety of Transcranial Magnetic Stimulation in the Acute Treatment of Major Depression: A Multisite Randomized Controlled Trial. Biological Psychiatry, 62(11), Article 11. https://doi.org/10.1016/j.biopsych.2007.01.018
O’Reardon, J. P., Solvason, H. B., Janicak, P. G., Sampson, S., Isenberg, K. E., Nahas, Z., McDonald, W. M., Avery, D., Fitzgerald, P. B., Loo, C., Demitrack, M. A., George, M. S., & Sackeim, H. A. (2007b). Efficacy and Safety of Transcranial Magnetic Stimulation in the Acute Treatment of Major Depression: A Multisite Randomized Controlled Trial. Biological Psychiatry, 62(11), 1208–1216. https://doi.org/10.1016/j.biopsych.2007.01.018
Osuch, E. A., Benson, B. E., Luckenbaugh, D. A., Geraci, M., Post, R. M., & McCann, U. (2009). Repetitive TMS combined with exposure therapy for PTSD: A preliminary study. Journal of Anxiety Disorders, 23(1), Article 1. https://doi.org/10.1016/j.janxdis.2008.03.015
Pell, G. S., Harmelech, T., Zibman, S., Roth, Y., Tendler, A., & Zangen, A. (2022). Efficacy of Deep TMS with the H1 Coil for Anxious Depression. Journal of Clinical Medicine, 11(4), 1015. https://doi.org/10.3390/jcm11041015
Petrosino, N. J., Wout-Frank, M. V. ’T, Aiken, E., Swearingen, H. R., Barredo, J., Zandvakili, A., & Philip, N. S. (2020). One-year clinical outcomes following theta burst stimulation for post-traumatic stress disorder. Neuropsychopharmacology, 45(6), 940–946. https://doi.org/10.1038/s41386-019-0584-4
Philip, N. S., Barredo, J., Aiken, E., Larson, V., Jones, R. N., Tracie Shea, M., Greenberg, B. D., & Van’T Wout-Frank, M. (2019). Theta-burst transcranial magnetic stimulation for posttraumatic stress disorder. American Journal of Psychiatry, 176(11), Article 11. https://doi.org/10.1176/appi.ajp.2019.18101160
Philip, N. S., Ramanathan, D., Gamboa, B., Brennan, M. C., Kozel, F. A., Lazzeroni, L., & Madore, M. R. (2023). Repetitive Transcranial Magnetic Stimulation for Depression and Posttraumatic Stress Disorder in Veterans With Mild Traumatic Brain Injury. Neuromodulation: Technology at the Neural Interface, 26(4), 878–884. https://doi.org/10.1016/j.neurom.2022.11.015
Pokorny, L., Besting, L., Roebruck, F., Jarczok, T. A., & Bender, S. (2022). Fearful facial expressions reduce inhibition levels in the dorsolateral prefrontal cortex in subjects with specific phobia. Depression and Anxiety, 39(1), 26–36. https://doi.org/10.1002/da.23217
Poppa, T., de Witte, S., Vanderhasselt, M. A., Bechara, A., & Baeken, C. (2020). Theta-burst stimulation and frontotemporal regulation of cardiovascular autonomic outputs: The role of state anxiety. International Journal of Psychophysiology, 149, 25–34. https://doi.org/10.1016/j.ijpsycho.2019.12.011
Raij, T., Nummenmaa, A., Marin, M.-F. F., Porter, D., Furtak, S., Setsompop, K., & Milad, M. R. (2018). Prefrontal Cortex Stimulation Enhances Fear Extinction Memory in Humans. Biological Psychiatry, 84(2), Article 2. https://doi.org/10.1016/j.biopsych.2017.10.022
Robinson, O., Krimsky, M., & Grillon, C. (2013). The impact of induced anxiety on response inhibition. Frontiers in Human Neuroscience, 7(March), 69. https://doi.org/10.3389/fnhum.2013.00069
Robinson, O., Letkiewicz, A. M., Overstreet, C., Ernst, M., & Grillon, C. (2011). The effect of induced anxiety on cognition: Threat of shock enhances aversive processing in healthy individuals. Cognitive, Affective & Behavioral Neuroscience, 11(2), 217–227. https://doi.org/10.3758/s13415-011-0030-5
Robinson, O., Overstreet, C., Allen, P. S., Pine, D. S., & Grillon, C. (2012). Acute tryptophan depletion increases translational indices of anxiety but not fear: Serotonergic modulation of the bed nucleus of the stria terminalis? Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 37(8), 1963–1971. https://doi.org/10.1038/npp.2012.43
Robinson, O., Vytal, K. E., Cornwell, B. R., & Grillon, C. (2013). The impact of anxiety upon cognition: Perspectives from human threat of shock studies. Frontiers in Human Neuroscience, 7(May), 203. https://doi.org/10.3389/fnhum.2013.00203
Rodríguez-Fornells, A., Riba, J., Gironell, A., Kulisevsky, J., & Barbanoj, M. J. (1999). Effects of alprazolam on the acoustic startle response in humans. Psychopharmacology, 143(3), 280–285. https://doi.org/10.1007/s002130050948
Ross, J. A., & Van Bockstaele, E. J. (2021). The Locus Coeruleus- Norepinephrine System in Stress and Arousal: Unraveling Historical, Current, and Future Perspectives. In Frontiers in Psychiatry (Vol. 11). https://www.frontiersin.org/article/10.3389/fpsyt.2020.601519
Rossi, S., Antal, A., Bestmann, S., Bikson, M., Brewer, C., Brockmöller, J., Carpenter, L. L., Cincotta, M., Chen, R., Daskalakis, J. D., Di Lazzaro, V., Fox, M. D., George, M. S., Gilbert, D., Kimiskidis, V. K., Koch, G., Ilmoniemi, R. J., Pascal Lefaucheur, J., Leocani, L., … Hallett, M. (2020). Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines. Clinical Neurophysiology, April, Article April. https://doi.org/10.1016/j.clinph.2020.10.003
Scaife, J. C., Langley, R. W., Bradshaw, C. M., & Szabadi, E. (2005). Diazepam suppresses the acquisition but not the expression of “fear-potentiation” of the acoustic startle response in man. Journal of Psychopharmacology, 19(4), 347–356. https://doi.org/10.1177/0269881105053285
Sheline, Y., Barch, D. M., Price, J. L., Rundle, M. M., Vaishnavi, S. N., Snyder, A. Z., Mintun, M. a, Wang, S., Coalson, R. S., & Raichle, M. E. (2009). The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences, 106(6), 1942–1947. https://doi.org/10.1073/pnas.0812686106
Suppa, A., Huang, Y. Z., Funke, K., Ridding, M. C., Cheeran, B., Di Lazzaro, V., Ziemann, U., & Rothwell, J. C. (2016). Ten Years of Theta Burst Stimulation in Humans: Established Knowledge, Unknowns and Prospects. Brain Stimulation, 9(3), 323–335. https://doi.org/10.1016/j.brs.2016.01.006
Sydnor, V. J., Cieslak, M., Duprat, R., Deluisi, J., Flounders, M. W., Long, H., Scully, M., Balderston, N. L., Sheline, Y. I., Bassett, D. S., Satterthwaite, T. D., & Oathes, D. J. (2022). Cortical-subcortical structural connections support transcranial magnetic stimulation engagement of the amygdala. Science Advances, 8(25), eabn5803. https://doi.org/10.1126/sciadv.abn5803
Teferi, M., Makhoul, W., Deng, Z.-D., Oathes, D. J., Sheline, Y., & Balderston, N. L. (2022). Continuous Theta Burst Stimulation to the Right Dorsolateral Prefrontal Cortex may increase Potentiated Startle in healthy individuals. Biological Psychiatry: Global Open Science. https://doi.org/10.1016/j.bpsgos.2022.04.001
Thomson, A. C., & Sack, A. T. (2020). How to Design Optimal Accelerated rTMS Protocols Capable of Promoting Therapeutically Beneficial Metaplasticity. Frontiers in Neurology, 11, 599918. https://doi.org/10.3389/fneur.2020.599918
Umscheid, C. A., Margolis, D. J., & Grossman, C. E. (2011). Key concepts of clinical trials: A narrative review. Postgraduate Medicine, 123(5), 194–204. https://doi.org/10.3810/pgm.2011.09.2475
van ’t Wout-Frank, M., Shea, M. T., Sorensen, D. O., Faucher, C. R., Greenberg, B. D., & Philip, N. S. (2021). A Secondary Analysis on Effects of Theta Burst Transcranial Magnetic Stimulation to Reduce Anger in Veterans With Posttraumatic Stress Disorder. Neuromodulation, 24(5), Article 5. https://doi.org/10.1111/ner.13256
Vos, T., Lim, S. S., Abbafati, C., Abbas, K. M., Abbasi, M., Abbasifard, M., Abbasi-Kangevari, M., Abbastabar, H., Abd-Allah, F., Abdelalim, A., Abdollahi, M., Abdollahpour, I., Abolhassani, H., Aboyans, V., Abrams, E. M., Abreu, L. G., Abrigo, M. R. M., Abu-Raddad, L. J., Abushouk, A. I., … Murray, C. J. L. (2020). Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. The Lancet, 396(10258), 1204–1222. https://doi.org/10.1016/S0140-6736(20)30925-9
Vytal, K. E., Overstreet, C., Charney, D. R., Robinson, O., & Grillon, C. (2014). Sustained anxiety increases amygdala-dorsomedial prefrontal coupling: A mechanism for maintaining an anxious state in healthy adults. Journal of Psychiatry & Neuroscience, 1–9. https://doi.org/10.1503/jpn.130145
White, D., & Tavakoli, S. (2015). Repetitive transcranial magnetic stimulation for treatment of major depressive disorder with comorbid generalized anxiety disorder. Annals of Clinical Psychiatry, 27(3), 192–196.
White, L. K., Makhoul, W., Teferi, M., Sheline, Y. I., & Balderston, N. L. (2023). The role of dlPFC laterality in the expression and regulation of anxiety. Neuropharmacology, 224, 109355. https://doi.org/10.1016/j.neuropharm.2022.109355
Wilkes, S., Ona, C., Yang, M., Liu, P., Benton, A., Lustik, M., & Coleman, J. (2020). Impacts of rTMS on Refractory Depression and Comorbid PTSD Symptoms at a Military Treatment Facility. Military Medicine, 185(9–10), e1420–e1427. https://doi.org/10.1093/milmed/usaa148
Zandvakili, A., Philip, N. S., Jones, S. R., Tyrka, A. R., Greenberg, B. D., & Carpenter, L. L. (2019). Use of machine learning in predicting clinical response to transcranial magnetic stimulation in comorbid posttraumatic stress disorder and major depression: A resting state electroencephalography study. Journal of Affective Disorders, 252(December 2018), Article December 2018. https://doi.org/10.1016/j.jad.2019.03.077
Zandvakili, A., Swearingen, H. R., & Philip, N. S. (2021). Changes in functional connectivity after theta-burst transcranial magnetic stimulation for post-traumatic stress disorder: A machine-learning study. European Archives of Psychiatry and Clinical Neuroscience, 271(1), Article 1. https://doi.org/10.1007/s00406-020-01172-5
Zangen, A., Moshe, H., Martinez, D., Barnea‐Ygael, N., Vapnik, T., Bystritsky, A., Duffy, W., Toder, D., Casuto, L., Grosz, M. L., Nunes, E. V., Ward, H., Tendler, A., Feifel, D., Morales, O., Roth, Y., Iosifescu, D. V., Winston, J., Wirecki, T., … George, M. S. (2021). Repetitive transcranial magnetic stimulation for smoking cessation: A pivotal multicenter double‐blind randomized controlled trial. World Psychiatry, 20(3), 397–404. https://doi.org/10.1002/wps.20905